Transform Your Practice. Schedule a Personalized DermaSensor Demo
Discover how seamlessly DermaSensor integrates into any practice setting, including standalone practices, health systems, walk-in clinics, concierge clinics, and more. Experience firsthand how our cutting-edge AI technology can elevate your practice.
Learn How to
Enhance Your Practice With DermaSensor
Designed with efficiency in mind, DermaSensor ensures rapid, objective test results without compromising quality. Its user-friendly interface makes it effortlessly accessible and easy to incorporate into the busy routine of medical offices. This powerful tool enhances and streamlines physicians’ management process for suspicious skin lesions and translates to tangible value for healthcare systems and payers.
See DermaSensor In action
Get an exclusive, live demonstration of the DermaSensor device. See how our AI-powered device performs skin cancer assessments with unmatched accuracy and ease.
Get Insider Knowledge
Learn how leading physicians are revolutionizing patient care with this new AI tool. Understand how DermaSensor enhances patient experiences, boosts efficiency, and transforms the landscape of skin cancer assessments.
Explore How DermaSensor is Transforming Skin Cancer Assessments
Tell us a bit about your practice so we can tailor your demo to your needs. Book your free personalized demo below.
As Featured In













Bridging the Gap in Skin Cancer Detection: Innovative Solutions for Rapid Diagnosis
Skin cancer is a pervasive and underdiagnosed health concern in the United States, exacerbated by the inefficiencies within the healthcare system. Primary care physicians, who are on the frontline to appropriately treat and triage a host of conditions, have had a lack of adequate diagnostic aids to assess skin lesions. There is an unmet need for tools to improve identification of lesions requiring referral, which may otherwise lead to delayed or missed detection of skin cancer.
Key Features
96% sensitivity for detection across all three common skin cancers¹
Proprietary algorithm yields instant assessment.
Ergonomically designed, wireless, and easy to grip.
Always on, ensuring readiness when needed.
How it Works?
Identify lesions suggestive of skin cancer on the patient
Apply handheld DermaSensor device to the lesion and initiate first recording
Five spectral recordings are taken from the skin lesion to complete one scan
DermaSensor's algorithm analyzes spectral data and delivers assessment in seconds
Technology
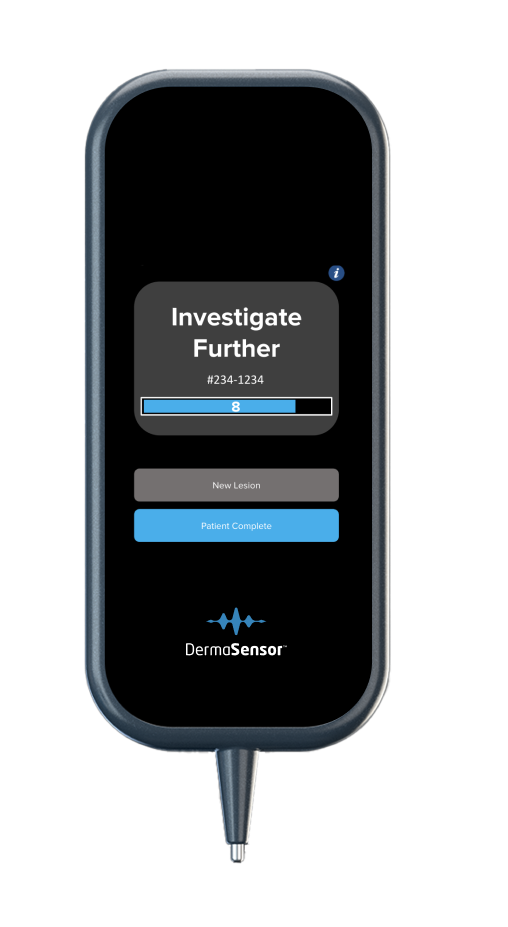
DermaSensor’s tip reflects and records quick bursts of light off the lesion’s cellular and sub-cellular content.
The light is analyzed by the built-in computer to provide information to help physicians assess skin lesions (including melanomas, squamous cell carcinomas, and basal cell carcinomas) to aid in a referral decision.
By examining the difference in light scattering, DermaSensor determines if the skin lesion output is “Monitor” or “Investigate Further,” with the latter result including a 1-10 confidence score for malignancy likelihood.
Elastic Scattering Spectroscopy (ESS) has been validated in 30+ publications on clinical studies for various cancer types. Many studies have shown ESS to provide information that is comparable to histopathologic assessment in the analysis of cellular microscopic structure.

University Of California, San Francisco
Discover what other practitioners are saying.