Introduction and Objectives

Skin cancer is the most common cancer worldwide and its most common types are basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and melanoma [1,2]. One in five Americans will develop skin cancer by the age of 70 and more than two people die of skin cancer in the U.S. every hour [3]. The mortality rate and morbidity of melanoma are highly associated with the stage at which the cancer is detected. Self-examination and GP detection play an important role in skin cancer management, as most melanomas have been shown to be detected by patients or primary care physicians [4].
Elastic-scattering spectroscopy (ESS) is an optical tissue sampling technique that can distinguish between normal and abnormal tissue in vivo by recording photons scattered back from chromophores. A handheld ESS device measures ESS spectra of skin lesions and classifies lesions as either malignant or benign with an output of “Investigate Further” or “Monitor”. The technology has been shown to have skin cancer sensitivity over 90% in various prospective, multi-centre studies [5,6,7,8]. This study aims to validate whether the use of an ESS point-of-care test can detect melanoma when dermatologists are evaluating lesions concerning for melanoma.
Materials & Methods
The handheld ESS device measures spectra of skin lesions and uses Convolutional Neural Networks to classify the lesion’s scanned properties against those of known malignant and benign lesions. The output of the ESS classifier is “Investigate Further” or “Monitor”. Additionally, for “Investigate Further” classified lesions, a score from 1 to 10 is provided which corresponds to the amount of spectral similarity a lesion has to malignant lesions in studies, with 10 representing the highest amount.
The algorithm was trained and validated with over 11,000 spectral scans from over 3,500 skin lesions, including histologically confirmed melanoma and non melanoma skin cancer (NMSC); as well as biopsied and unbiopsied benign lesions, as diagnosed by board-certified dermatologists [6,7]. The device is intended to act as an adjunctive tool for lesions that are suspicious of melanoma or NMSC.
This study consisted of ten dermatology study centers scanning lesions that they found concerning for melanoma. All dermatologists were blinded to the device results. Gold-standard comparison for performance of the device and dermatologists was the biopsy result with multiple dermatopathologist review when consensus was not reached during the primary review process. High resolution digital images and the patient’s clinical information, including prior skin cancer history, risk factors and physical examination results, were recorded for each case. After clinical evaluation, dermatologists reported their diagnosis and confidence level, which provided the physician comparison data. The results evaluated were sensitivity, specificity, Negative Predictive Value (NPV, and Positive Predictive Value (PPV) for melanoma, melanoma + severely atypical nevi, and all high-risk lesions. Area Under the Curve (AUC) was also calculated and modelled and compared between the study dermatologists and the device performance.
Results
A total of 311 patients with 440 biopsied lesions were evaluated by the study dermatologists, device and dermatopathology results. Most patients were male (54%), white (98%) and non-Hispanic (96%) and Fitzpatrick skin type II (53%) or III (20%). Lesions were most commonly described as flat (84%), pigmented (96%) and with a median length of 5mm.
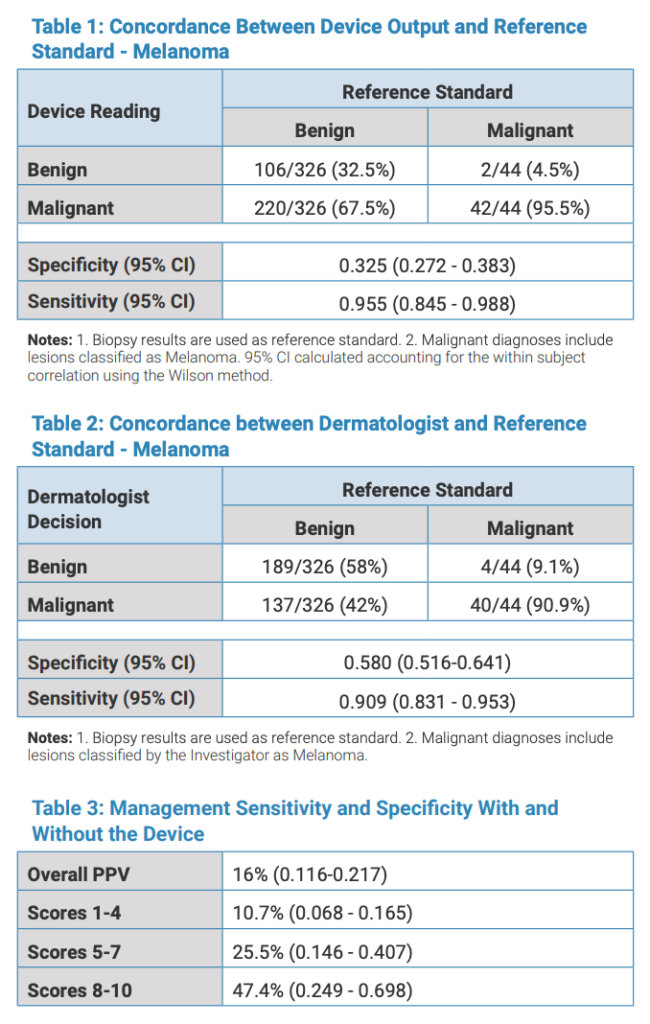
Biopsy revealed 114 high-risk lesions with 44 melanomas, 44 severely atypical nevi (SAN) and 26 NMSC. Cancer prevalence was 26% (Number Needed to Biopsy (NNB): 4). Diagnostic sensitivity of the device was 96% (95% CI: 0.845 – 0.988) for melanoma alone, 91% for melanoma and SAN (95% CI: 0.831 – 0.953) and 93% (95% CI: 0.834 – 0.971) for all malignant lesions (Table 1). Overall device specificity was 33% (95% CI: 0.272-0.383). Dermatologist’s sensitivity was 91% (95% CI: 0.806 – 0.960) for melanoma, 72% (95% CI: 0.613 – 0.800) for melanoma + SAN (Table 2).
For prevalence assumptions like those seen in general dermatology practice, the predicted NPV of the device is 97.3% with a PPV of 11.8% (Table 4).
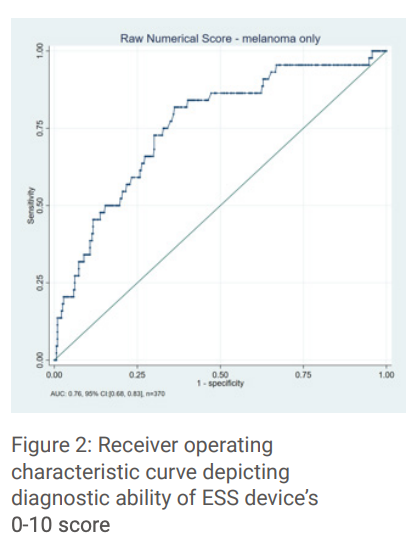
The AUC of the ESS device for melanoma detection was 0.76 compared to the dermatologists of 0.75. Figure 2 demonstrates the AUC of the device for scores 0-10. The negative result of “Monitor” is represented by 0.
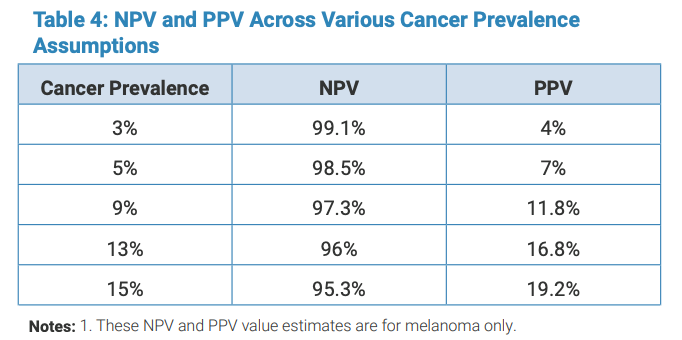
The overall NPV for a monitor result was 98.1% for melanoma. The overall PPV for a positive device result was 16%. When looking at spectral score groupings, the PPV increased for higher scores, with scores of 1-4 having a PPV of 11%, scores 5-7 with a PPV of 26% and scores of 8-10 having a PPV of 47%. (Table 3).
Discussion and Conclusion
The use of the handheld ESS device by physicians, in addition to clinical evaluation, may improve melanoma detection. The device was able to identify 96% of melanomas when compared to dermatopathology results. In addition, the high NPV of ~98% can provide confidence in ruling out melanoma. When presented with a high score (8-10), the PPV was 47% for melanoma which equates to a NNB of 2, meaning approximately 50% of scores 8-10 may be melanoma.
The high concordance of the handheld device with dermatopathology may help alleviate patient and dermatologist concern for benign lesions mimicking melanoma, particularly for nevi that do not merit re-excision.
When compared to other tools and the current gold-standard available for visually assessing skin lesions (i.e. ABCDEs), the ESS device has AUC similar to dermatologists, high sensitivity for malignancy, and reasonable specificity for ruling out suspicious yet benign lesions biopsied by dermatologists, thereby providing important objective information to providers. While this study was conducted by melanoma specialists, given the device’s similar overall performance to these specialists and its simple, non-invasive use, there is potential for the device to be used to help rule in or out lesion referrals for primary care physicians.
References
- Cancer Facts and Figures 2021. American Cancer Society. https:// www.cancer.org/content/dam/cancer-org/research/cancer-facts-andstatistics/annual-cancer-facts-and-figures/2021/cancer-facts-andfigures-2021.pdf. Accessed January 13, 2021.
- Skin Cancer Statistics 2021. World Cancer Research Fund. https:// www.wcrf.org/dietandcancer/skin-cancer-statistics/Accessed September 1, 2021.
- Mohan SV, Chang AL. Advanced basal cell carcinoma: epidemiology and therapeutic innovations. Current dermatology reports. 2014 Mar 1;3(1):40- 5.
- Fahradyan, A., Howell, A. C., Wolfswinkel, E. M., Tsuha, M., Sheth, P., & Wong, A. K. (2017, December). Updates on the management of nonmelanoma skin cancer (NMSC). In Healthcare (Vol.
- Rodriguez-Diaz E, et al. Optical Spectroscopy as a Method for Skin Cancer Risk Assessment. Photochem Photobiol. 2019;95(6):1441-1445.
- Benvenuto-Andrade C, Manolakos D, Cognetta AB. Safety and Effectiveness of Elastic Scattering Spectroscopy and Machine Learning in the Evaluation of Skin Lesions. Poster Presentation, World Congress of Teledermatology, Nov 2020
- Manolakos, D. Rabinovitz, H. Geisse J, Bonning M. Rodriguez-Diaz, E. Bigio I. Cognetta, A. Clinical Validation of a Handheld Elastic Scattering Spectroscopy- Artificial Intelligence Device. Podium Presentation at AAD Innovations Academy, Jul 21-24th 2022.
- Tepedino M., Balthazar D., Hucks, C., Zeitouni, N. Use of Elastic-scattering spectroscopy on Patient-Selected Lesions that are Concerning for Skin Cancer. Poster Presentation at American Dermoscopy Meeting, Jun 29- Jul 2, 2022
Disclosures
This study was supported by a grant from DermaSensor Inc.


